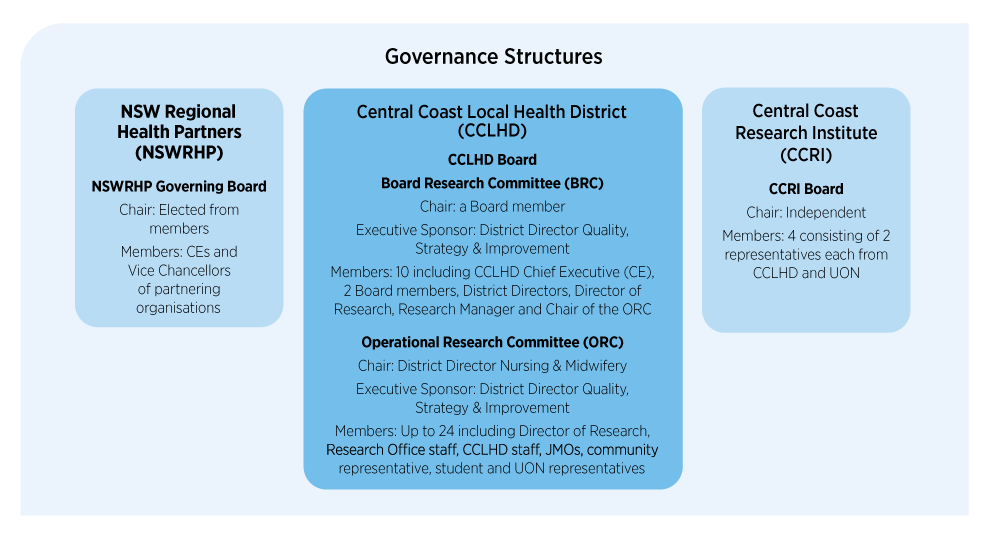
All research involving humans conducted within the NSW public health system must be ethically and scientifically reviewed and approved by a Human Research Ethics Committee (HREC) in accordance with the National Health and Medical Research Council’s National Statement on Ethical Conduct in Human Research (2023).
In addition, all human research that takes place in NSW public health organisations, or that requires support from a NSW public health organisation in the form of access to participants, tissue or data, requires site authorisation. Site authorisation must be reviewed and authorised by the Chief Executive or their delegate before commencement.
Applications for ethics approval and site authorisation are submitted via the Research Ethics and Governance Information System (REGIS). However, applications for Access Requests are currently submitted directly to the CCLHD Research Office by email following ethics approval.
There are separate approval processes in place at CCLHD for some Lower Risk (LR) research projects and for Quality Improvement (QI) projects. Please contact the CCLHD Research Office if you need guidance in selecting the appropriate pathway for authorisation of your research or quality project.
How to Submit a Site Specific Assessment
Ethics Submissions
Before conducting research at CCLHD you will need to obtain approval from a Human Research Ethics Committee (HREC). You have the choice of using any one of the certified NSW Health Ethics Committees from the Ethical & Scientific Review list. Please note we do not have an Ethics Committee at CCLHD but are able to provide guidance. Once ethical approval is granted, you will need to submit a Site Specific Assessment.
For standard templates (e.g. protocol or participant information/consent forms) please contact your chosen HREC office or view its website.
For clinical trials support, guidance and Information please visit:
![]() Office for Health and Medical Research (OHMR) clinical trials NSW
Office for Health and Medical Research (OHMR) clinical trials NSW
Governance (Site Specific Assessment) Submissions
In addition to ethical and scientific review, all human research that takes place in NSW public health organisations, or that requires support from a NSW public health organisation in the form of access to participants, tissue or data, must be reviewed and authorised by the organisation’s Chief Executive, or their delegate before commencement. The project must not commence until the applicant has received notification of documented authorisation.
Site authorisation enables NSW public health organisations to:
- Ensure the proposed research project complies with appropriate ethical, scientific, regulatory and professional standards.
- Consider whether the project should be conducted at and supported by the organisation, and/or whether the provision of access to participants, their tissue and/or data should be supported.
- Be aware of all research taking place at sites under their control.
Please refer to our Checklist for Researchers to help when submitting your site governance (STE) application to REGIS. This checklist has been developed by representatives from nine health service organisations across NSW, agreeing on common items to create a more consistent approach across the LHDs.
![]() Checklist for Researchers (Word 690 KB)
Checklist for Researchers (Word 690 KB)
There are two pathways for site authorisation in NSW, depending on the research activities:
- Site Specific Assessment
- Access request review
For more information, please see the OHMR site authorisation information:
The wording in the local Participant Information Sheet and Consent Form (PISCF) should be the same as that approved by the lead HREC for the Master PISCF.
Add CCLHD Research Office details under “Complaints about this research” on the Participant Information Sheet in addition to the Ethics Office details, as follows:
“This study has been authorised by the Central Coast Local Health District (CCLHD) Research Office for conduct at [Site name/s]. Any person with concerns or complaints about this study may also contact the CCLHD Research Manager on 02 4320 2085 or email: CCLHD-Research@health.nsw.gov.au, and quote the REGIS reference number 20xx/STExxxxx
REGIS
All Site Specific Assessment Applications (SSA) are submitted using REGIS. The REGIS website has a library of helpful Quick Reference Guides to assist with the application process.
Please note that REGIS is not managed by CCLHD Research Office, and we cannot provide technical support beyond basic queries. If you are experiencing technical difficulty with the REGIS website, please contact REGIS Help Desk on 1300 073 447. The CCLHD Research Office provides Research Governance review for the following sites:
- Gosford Hospital
- Wyong Hospital
- Woy Woy Hospital
- Long Jetty Healthcare Centre
- CCLHD Community Health Centres
If you have an SSA application that includes two or more of these sites, please contact the Research Office to discuss prior to submission to ethics.
CCLHD Institution Details
The legal institution details should be used on the following forms:
1. Clinical Trial Research Agreements (CTRA)
Name of Institution: Central Coast Local Health District
Address: Holden Street, Gosford NSW 2250
ABN: 88 523 389 096
Contact for Notices: “CCLHD Study Coordinator”
Fax for Notices: “CCLHD Study Coordinator fax number or email”
Phone Number: “CCLHD Study Coordinator phone number”
2. Medicines Australia Form of Indemnity (HREC only and Standard)
To: Central Coast Local Health District, Gosford Hospital, Holden Street Gosford NSW 2250 (“the Indemnified Party”)
3. Clinical Trial Notification (CTN) – for investigator initiated research projects (not commercially sponsored)
Name of approving authority: Central Coast Local Health District Research Office
Approving authority Contact Officer: “Name of Research Manager”
Position: Research Manager
Contact Phone: 02 4320 2085
Contact email: CCLHD-Research@health.nsw.gov.au
Head of Department Support
All research applications require evidence of support (signatures) from the relevant Head/s of Department/s, Director or General Manager to whom each investigator at the site is responsible. The Principal Investigator is also responsible for obtaining signatures from the relevant heads of supporting departments, whose departments will be involved in the research, including the nominated authorities for data provision (i.e. data custodians for data sets required to be accessed as a part of the research); as well as student supervisors for participating investigators.
Please be sure to engage with Heads of Department (or equivalent) to discuss the particulars of the project (especially any resources that will be impacted) before sending a request for support via REGIS. If you are unsure of the name of the head of a department, please contact the Research Office for assistance.
External Researchers
Are you employed by another LHD, University (staff or student) or external organisation and wish to conduct research activity at CCLHD?
External researchers who wish to conduct research within CCLHD may be required to provide additional information, as outlined in the table
below.
For any queries about this process, please contact the CCLHD Research Office to discuss.
| Documentation | Employee of a NSW Health LHD other than CCLHD | Medical student on placement at CCLHD | Employee or student at a university or employee at an external organisation |
|---|---|---|---|
| Letter from PI* or supervisor outlining scope of onsite activity and duration |
Yes | Yes | Yes |
| Signed form |
No | No | Yes |
| Copy of scope of liability insurance, along with certificate of currency | No | No | Yes |
| Copy of photo ID** | Yes, Staff ID | Yes, Staff ID | Yes |
| Copy of National Police Check (without fingerprint) at investigators expense. or current Working with Children Check |
No | No | Yes |
| Copy of Curriculum Vitae (CV) | No | Yes | Yes |
*if the PI or supervisor is not a CCLHD employee this letter should be from the Head of the Department (HOD) who will be taking the responsibility of hosting the external researcher. If the project is submitted via REGIS this is not required as HOD support is provided via endorsement.
** If you do not wish to upload photo IDs with your project application, please advise the Research Office team to organise an alternative.
If your project will require direct contact with participants (patients, family/carers or staff) or access to CCLHD data, please contact the CCLHD
Establishment team: CCLHD-Establishment@health.nsw.gov.au
Fees and Payments
Please be advised that all new research projects reviewed by the CCLHD Research Office will incur a Governance Review Fee in order to provide sufficient funds to support the administration of research governance. Fees vary depending on the type of submission, and the study sponsor/funding type.
Clinical trial fees are mandated by the NSW Ministry of Health, Policy Directive: Fee Schedule for Research Ethics and Governance Review of Clinical Trial Research (PDF).
Once your project has been submitted, the Research Office will provide a method of payment form to be completed prior to authorisation.
Post Authorisation
Submitting an Amendment
The Principal Investigator at a site is responsible for notifying the Research Governance Officer (RGO) of all HREC approved amendments to a research project. Please note that amendments cannot be reviewed by the RGO without evidence of relevant HREC approval.
Amendments include changes to investigator brochures, protocol, consent forms, extension requests to ethical approval, addition of site requests and change in personnel requests (CPI/PI only). All amendments must be submitted via REGIS for Site Specific Amendments. For further guidance please see:
![]() Site Amendment – Completing & Submitting (PDF)
Site Amendment – Completing & Submitting (PDF)
Submitting Milestone (formerly known as Annual Report) Reports
Milestone reports (formerly known as annual or final reports) are due on the anniversary of the ethics approval. For further guidance please see:
![]() Submitting a Governance Milestone (PDF)
Submitting a Governance Milestone (PDF)
Any updates to a studies Certificate of Insurance can also be submitted via a milestone.
Changes to Research Personnel
If there are any changes to research personnel on your study, please submit these changes via a general amendment. If the Principal Investigator (PI) of the SSA Application is changing, this will need to be approved by the HREC prior to being submitted. For further guidance please see:
![]() Site Amendment – Completing & Submitting (PDF)
Site Amendment – Completing & Submitting (PDF)
Safety Reporting for Clinical Trials
Please note safety reports are submitted in the same way as amendments.
For further guidance on how to submit an amendment or safety report in REGIS please see:
![]() Submitting a Clinical Trial Safety Report (PDF)
Submitting a Clinical Trial Safety Report (PDF)
Use of Logos
We ask that researchers please use the CCLHD logo on all participant consent forms. If you do not have a copy of the logo, please contact our Design and Print team at CCLHD-DesignPrint@health.nsw.gov.au.
Helpful Links
![]() Standard Clinical Trial Research Agreements
Standard Clinical Trial Research Agreements
![]() Indemnity Forms (HREC review only and standard) and Compensation Guidelines
Indemnity Forms (HREC review only and standard) and Compensation Guidelines
Non-HREC review of Lower Risk (LR) Research Applications
The Non-Human Research Ethics Committee (HREC) review of Lower Risk Research Application form is used for projects conducted within Central Coast Local Health District (CCLHD) by CCLHD staff that meet the requirements to be considered as exempt from HREC review for lower risk Research in line with NSW OHMR Guideline for Low and Negligible Risk Research. The application form can be found on the CCLHD Research Office Intranet page.
Applications that involve more than one LHD do not fit within this review process and will require ethical review.
CCLHD encourages research and is responsible for ensuring that such activities follow the policies and guidelines around ethical considerations. These considerations include whether the people involved (e.g. participants, staff or the community) will be exposed to any risk, burden, inconvenience or possible breach of their privacy.
If you feel your study fits the Non-HREC review of Lower-Risk Research criteria, please contact the CCLHD Research Office to discuss further.
Research Support Services & Resources
Education & Training
To promote and support research at CCLHD, our Research Office staff provides opportunities to attend workshops, connect with mentors, find collaborators, network and discuss their projects with a broader audience. We partner with Library Services, the Nursing & Midwifery Directorate and university partners to provide workshops and master classes.
Stay up to date with upcoming education and training via our mailing list.
Data Management & Biostatistics
Biostatistics information
CCLHD staff and students can access advice and support for Research and Quality Improvement (QI) projects or grant applications from a Biostatistician (from HMRI) by contacting the Research Office on CCLHD-Research@health.nsw.gov.au.
Please advise the name/s of the department/s involved in the project or QI activity and the title of the project or grants scheme.
Accessing Patient Records & Data
Health Information Services provides support to CCLHD Research Office and Researchers.
- CCLHD Research Office seek endorsement for Quality Improvement requests when medical records are to be accessed.
- Support Researchers – Organise access to eMR – Powerchart to view medical records. We require staff name, employee number and area of research i.e. Emergency Department.
Contact Health Information Services via email at CCLHD-HISDataQuality@health.nsw.gov.au.
Library
CCLHD library staff provide information services for all CCLHD staff including help with searching, training, literature searches and reviews and inter library loans. The libraries have computers, printers and photocopiers for all staff and CCLHD based University of Newcastle medical students to use. The library can provide one on one and small group training on using CIAP, CINAHL, Endnote, Returning to Study, using the library catalogues and searching databases. Give them a call on 4320 3856 (Gosford) or 4394 9023 (Wyong) or visit them online for more information and access to more online resources and the library catalogue.
Student Support
The University of Newcastle Central Coast Clinical School is committed to providing world class education and research. Its location and facilities afford students a diverse clinical experience with access to placements in both Gosford and Wyong hospitals, as well as a number of smaller public and private facilities.
![]() University of Newcastle Central Coast Clinical School
University of Newcastle Central Coast Clinical School
With dedicated staff, the University of Newcastle Central Coast Clinical School is able to provide a high level of support to all students including dedicated support for Indigenous students.
From 2021, 32 students who accept an offer to study the Joint Medical Program (JMP) at the University of Newcastle will be located at The University of Newcastle Central Coast Clinical School for their first three years of study. Prospective students will have an opportunity to lodge an expression of interest for allocation to the Central Coast Clinical School when they submit their University of Newcastle application.
All JMP students, enrolled at either University of Newcastle or University of New England, will be able to nominate placements at the Central Coast Clinical School in the latter years of the program.
NHMRC Consumer and Community Engagement Tools
The National Health and Medical Research Council (NHMRC) understands the importance of engaging with consumers and the community on its role and activities, whilst also providing leadership and guidance to the health and medical research sector on the meaningful engagement of consumers throughout all stages of research and health care.
![]() Consumer and community engagement
Consumer and community engagement
Aboriginal Health and Medical Research Council (AH&MRC)
The Aboriginal Health and Medical Research Council (AH&MRC) assists Aboriginal Community Controlled Health Services (ACCHSs) across NSW to ensure they have access to an adequately resourced and skilled workforce to provide high-quality health care services for Aboriginal communities.
In 1996, the NSW Aboriginal Health Resources Cooperative, later known as the Aboriginal Health & Medical Research Council (AH&MRC), established the AH&MRC Human Research Ethics Committee (Ethics Committee) to embed ethical research principals and better outcomes for Aboriginal people and their communities. Prior to the creation of the committee, many health and medical research projects involving Aboriginal people were deemed to be invasive, inappropriate, unnecessary, and undertaken without consultation or approval by Aboriginal people and communities.
In line with CCLHD’s commitment to Indigenous peoples and culture, researchers will exercise sensitivity in any research associated with Aboriginal and Torres Strait Islander peoples. Researchers will comply with the guidelines under the NSW Aboriginal Health and Medical Research Committee (AH&MRC Ethical Guidelines: Key Principles (2020) V2.0) and also the NHMRC’s Guidelines for Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities (2018).
If your project is focused on Aboriginal and Torres Strait Islander health, please contact the AH&MRC to confirm if you require review by the AH&MRC Ethics Committee.